Spiking Model In Vivo Neurophysiology
The animals employed for neurophysiological recording were adult, male Long-Evans rats acquired at 4 months of age. The animal protocols used were approved by the University of Kansas Medical Center Institutional Animal Care and Use Committee, and the experiments were performed in compliance with the Guide for the Care and Use of Laboratory Animals. Each rat was singly housed in a transparent cage and provided with food and water ad libitum. The room was kept on a 12-h:12-h light/dark cycle, and ambient temperature was maintained at 22 °C. The in vivo neuronal recordings were collected from ketamine/xylazine-anesthetized rodents within the left-sided rostral forelimb area (RFA). A contact site in the MEA yielding neural spiking recording with moderate spontaneous activity rate (4-10 spikes/s) was identified and used as a reference (trigger) channel. The triggered stimulus pulse was a single 60 μA biphasic,cathodal-leading pulse (200 μs positive, 200 μs negative) delivered to a cortical site in the forelimb-responsive (FL) area (Figure 3.1, Processing Unit). Latency between spike detection in RFA and delivery of a stimulus pulse in either FL or barrel field (BF) (Figure 3.1, Stimulation Unit) was preceded by a 10 ms delay (2.5 ms spike processing time, 7.5 ms imposed delay). The extracellular signals were acquired within RFA using a four-shank, sixteen-contact site Michigan-style electrode with 1-1.5 MΩ impedance at each site (A4x4-5mm-100-125-703-A16, NeuroNexus, Ann Arbor, MI). The probe was placed with the deepest contact at 1600 μm below the cortical surface (Figure 3.1, Recording Units). An active, unity-gain head stage was attached to the probe, and connected through a preamplifier to the recording system Tucker-Davis Technologies (TDT), Gainesville, FL, USA). Electrical stimulation was applied through a single contact on a single-shank, sixteen-contact electrode with an impedance of about 200 kΩ (e.g. contact point 6, activated A1x16-5mm-100-703-A16, NeuroNexus Technologies, Ann Arbor, MI, USA), and was delivered using a stimulus isolator and passive head stage (MS16 Stimulus Isolator, TDT). Multichannel recordings of four experiments were stored, which started with a ten-minute period of no stimulation followed three one-hour periods of stimulation separated and followed again by ten-minute periods of no stimulation, for a total of approximately three hours and forty minutes of recorded data) as depicted in Figure 3.1.
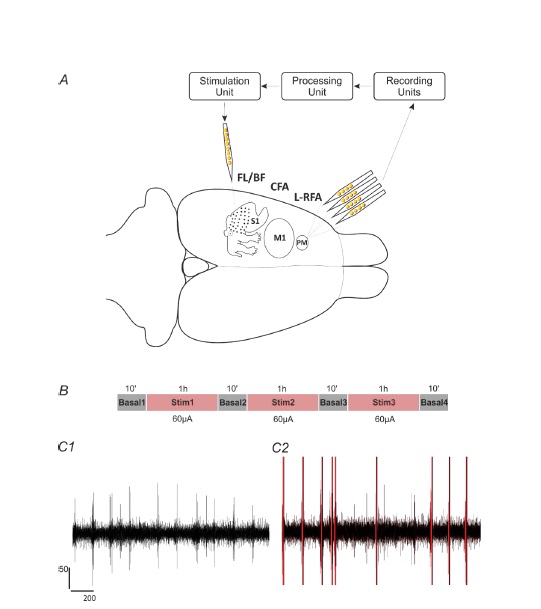
Figure 3.1. Recording and stimulation experimental paradigm. A. Methodology described on an illustration highlighting the cortical reference and stimulation regions under study. Extracellular recordings were acquired with a four-shank, 16-contact microelectrode array with intra-shank contact distances of 100 μm and cross-shank distances of 125 μm. The signals were acquired using the TDT recording system (Recording unit), processed to detect the single-unit activity (Processing unit), from which a user selected a reference neuron employed to trigger a stimulation pulse to a site on a single-shank microelectrode (Stimulation unit). B. Recording sessions consisted of three one-hour intermittent periods of stimulation separated each by ten-minute periods of no stimulation. C1. Extracellular recording during basal period lasting 2s, and filtered ~300-3k Hz. C2. Extracellular recording during stimulation period lasting 2s, and filtered ~300-3k Hz. Red lines represent each stimulation pulse artifact.


