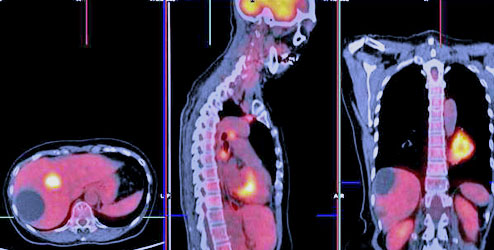
Radiotracer
Radiotracers (radiopharmaceuticals) are combinations of a drug or a biologically active compound which acts as a vehicle for targeted delivery and a radioisotope for localization purposes. The use of radiotracers in nuclear imaging is a non-invasive method for accessing cellular functions, which involves identifying useful biomarkers, specific characteristics of a disease, or biochemical processes that need be measured.
Some general properties of a radiotracer are :
The effective half life of the radiopharmaceutical should be appropriate to serve the imaging purpose and to lessen radiation exposure of the patient. Thus if tel is the effective half life , tbl is the biological half life and tpl is the physical half life, then
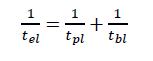
formula 1
The resulting emission of either γ or beta from the radionuclide should have the proper energy for the detector (e.g. 511 keV for PET and between 100-250 keV for other γ camera imaging devices).
A high probability of emissions per decay, that is, a sufficient abundance of the signal to be detected.
A high target to non-target ratio . This helps eliminate most of the background signal and provide more details of the target site. Otherwise it will be almost impossible to distinguish the actual pathology from the background, especially in therapeutic procedures, in order to prevent the patient from excessive radiation dose.
The radiopharmaceutical should be easily compounded and free of any physiological effect uninterested.
The radiolableling should not be affected by the carrier pharmaceutical as this could affect the biodistribution or the compound.
There are several radiotracers used in nuclear imaging today which are specifically chosen to fit both the patient and the test in question. We will focus on two specific radioisotopes F-18 and Cu-64, which are very important and desirable constituents of a vast majority of radiopharmaceuticals today.
Cu (T ½ = 12.7 hours; β+, 0.653 MeV [17.8 %]; β−, 0.579 MeV [38.4 %]) is generally used as a positron emitter for PET. For example, Cu is used as radioisotope in 64Cu-diacetyl-bis(N4-methylthiosemicarbazone)(64Cu-ATSM) especially for the diagnosis of hypoxia. The 12.7-hours half-life of Cu-64, in a practical sense, provide sufficient time for imaging of the target. Due to the versatility of Cu-64, there has been an abundance of novel research in this area over the past 20 years, primarily in the area of PET imaging, but also for the targeted radiotherapy of cancer. Recently, the chelator-free [Cu]-copper sulfide (CuS) nanoparticles are a good candidate for image-guided photothermal therapy.
18: F ( T½ = 109.7 min; β+ , 0.635MeV [97%]) on the other hand is a major constituent of several radiophamaceuticals. A Radiotracer like 2-Deoxy-( 18 F) fluoro- D-glucose (FDG) is the most common radiotracer used in PET to stage cancer and locate metastasis in many regions of the body. FDG is analogous to glucose and is taken up by living cells through the normal glucose pathway. Tumor imaging with FDG relies on the fact that malignant cells show higher metabolic rates of glucose absorption than normal tissue and therefore take up greater amounts of FDG. The radionuclide 18F has a high specific activity, and emit positrons with low energy is ideal for PET scans.

Radiotracer