Genetics
Population-based epidemiological surveys and twin studies greatly support the idea that MO is produced by genetic and environmental influences (Goadsby, 2013). Epidemiological evidence demonstrates methyltetrahydrofolate reductase C677T gene mutation is over-prevalent in people with MA (Goadsby, 2013). Other population-based epidemiological information indicates that there is no additional risk in cognitive function for migraineurs when compared to sex- and age-matched controls.
About 50% of reported families who experience migraine have Familial Hemiplegic Migraine I (FHM I) which are mutations in chromosome 19p13 (Goadsby, 2013). These mutations comprise Cav2.1 (P/Q)-type voltage-gated calcium channel CACNA1A gene (Ophoff et al., 1996). Chromosome 19-linked and unlinked FHM families are similar clinically. Cerebellar ataxia is one clinical trait that does associate with chromosome 19 mutations in about 50% of cases, but is not connected to unlinked families. Also, chromosome 19-linked families may have migraine attacks generated by minor head trauma or correlated with coma, but these differences are less prominent than cerebellar ataxia. ATP1A2 mutations have been found to cause approximately 20% of FHM families and have been designated as FHM II (De Fusco et al., 2003) in which epilepsy also occurs (Jurkat-Rott, 2004). The normal ATP1A2 gene codes for Na+/K+ ATPase (De Fusco et al., 2003). The mutation causes a smaller electrochemical gradient for Na+ ions that decreases or shuts down astrocystic glutamate transporters, resulting in an
accumulation of synaptic glutamate (Goadsby, 2013). The FHM III missense mutation, Q1489K in SCN1A, is described in 3 German families (Dichgans, 2005). A highly conserved glutamine is replaced by lysine in an area of the voltage-gated sodium channel that participates in the hyperpolarization after membrane depolarization. Thus, this mutation prevents a rapid closure which creates a continuous influx of sodium (Goadsby, 2013). As indicated by genetic studies, migraine, particularly MA, may be a channelopathy such as dysfunctional noradrenergic and serotonergic channels in the locus coereleus (LC) and dorsal raphe nucleus (DRN), respectively (McMahon et al., 2013). Further, channel dysfunction has been connected to aura in a study that created a knockin mouse with a CACNA1A missense mutation that caused an increased susceptibility to CSD by a reduced threshold and increased velocity of CSD (van den Maagdenberg et al., 2004). These knock-in mouse models were employed in other studies to observe nociceptive activation of trigeminal dura utilizing Fos protein expression. In contrast to wild-type animals, second order neuronal activation was reduced and Fos protein expression was increased in particular thalamic nuclei (Goadsby, 2013).
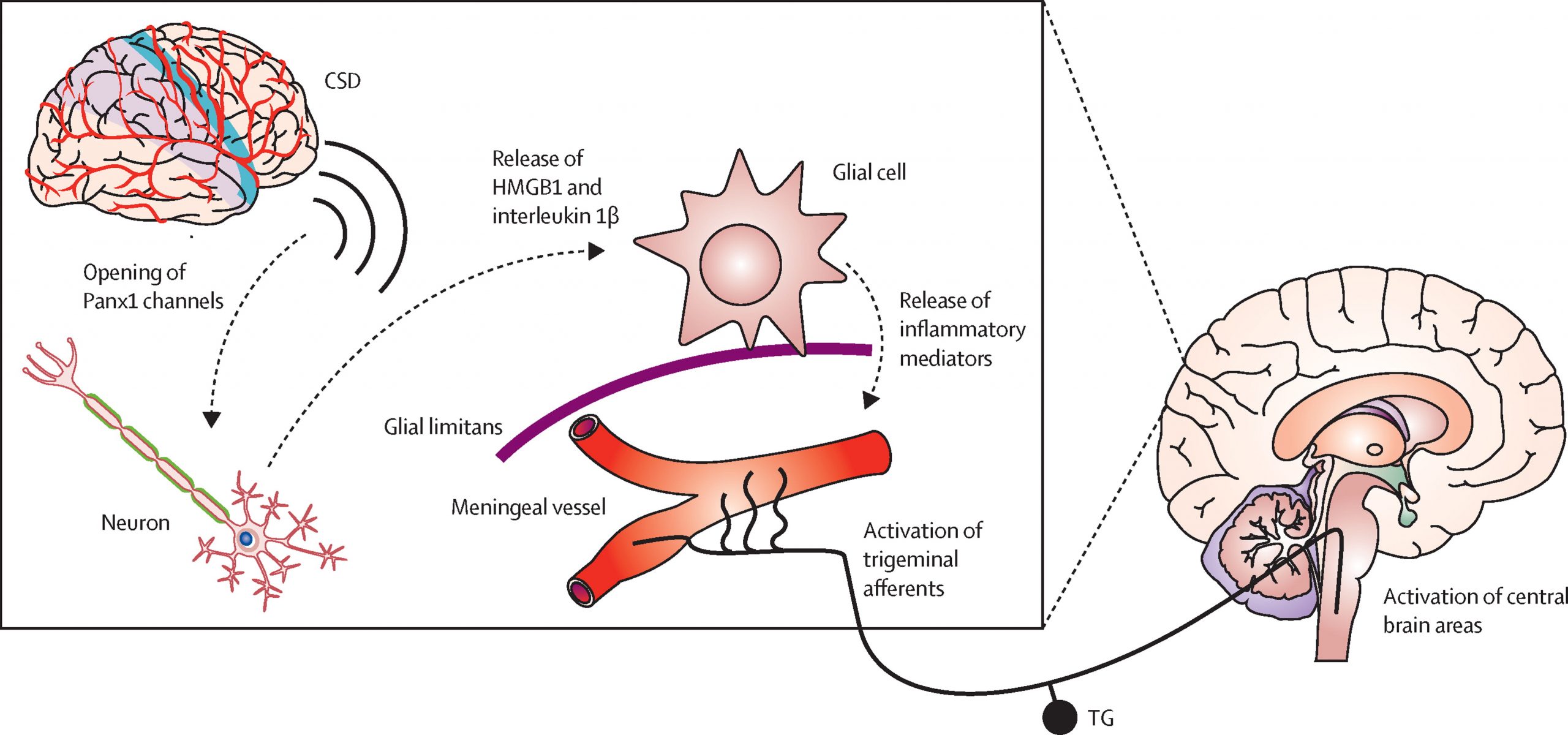
Genetics