Information processing in the brain is carried out by large groups of interconnected neurons. Neurons are the cells responsible for encoding, transmitting, and integrating signals originating inside or outside the nervous system. The transmission of information within and between neurons involves changes in the resting membrane potential, when compared to the extracellular space. The inputs one neuron receives at the synapses from other neurons cause transient changes in its resting membrane potential, called postsynaptic potentials. These changes in potential are mediated by the flux of ions between the intracellular and extracellular space. The flux of ions is made possible through ion channels present in the membrane. The ion channels open or close depending on the membrane potential and on substances released by the neurons, namely neurotransmitters, which bind to receptors on the cell’s membrane and hyperpolarize or depolarize the cell. When the post synaptic potential reaches a threshold, the neuron produces an impulse. The impulses or spikes, called action potentials, are characterized by a certain amplitude and duration and are the units of information transmission at the interneuronal level.
brain-machine interfaces to help paralyzed patients and development of biologically inspired computational devices.
Brain-Machine Interface:
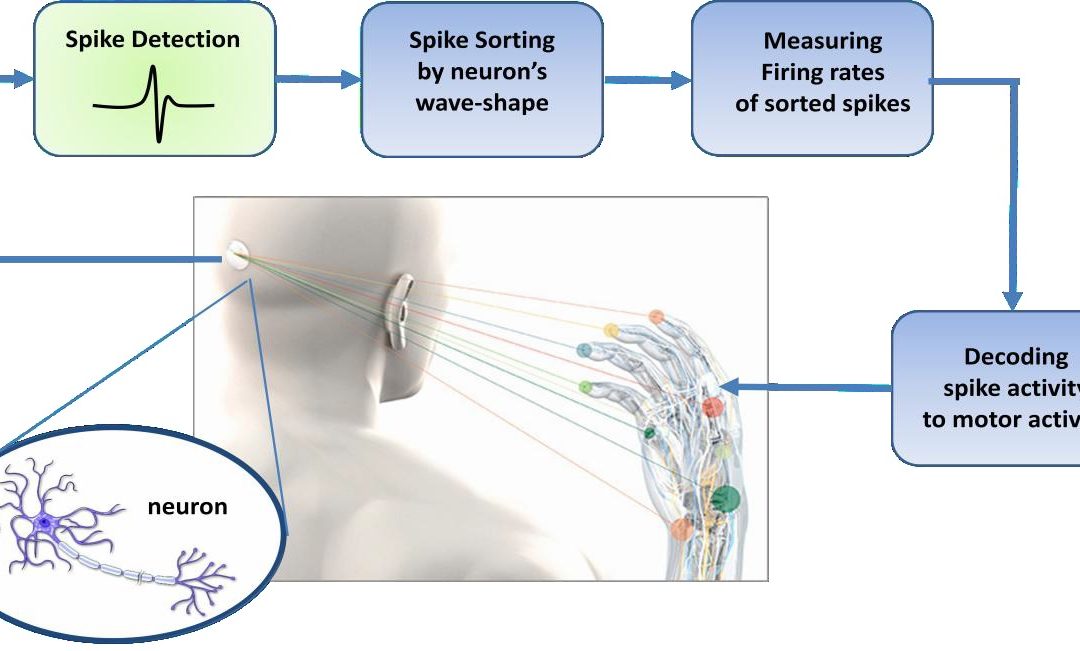
Extracting motor control signals from the firing patterns of populations of neurons and using these control signals to reproduce motor behaviors in artificial actuators are the key operations of a brain-machine interface. The typical neural signal processing pathway as shown in Fig.1.1 is designed to measure the instantaneous frequency of neural action potentials, or spikes. Since any given electrode may sense spikes from multiple neurons, it is typically necessary to sort all detected spikes by waveshape (i.e.by neuron). Firing rates of sorted spikes are typically measured by moving average; these rates can then be used by “decoding” algorithms which use statistical models to correlate spiking activity with behavioral or motor activity in the subject.
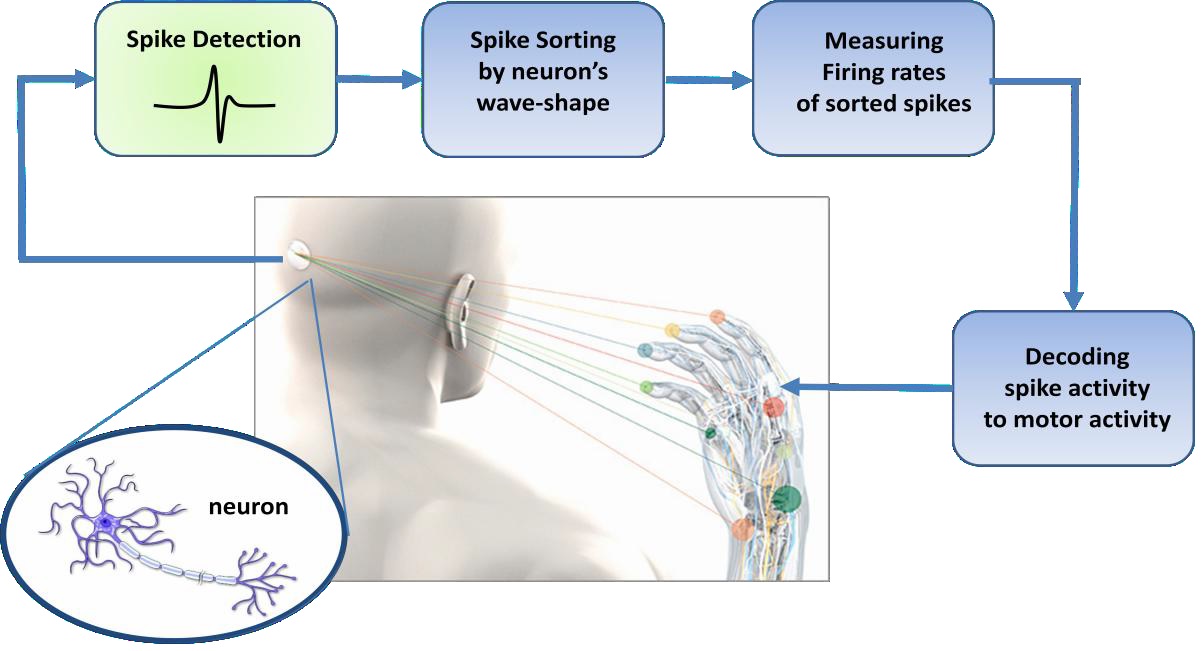
Fig.1.1 Block diagram of the typical pathway of brain machine interface
Hence invasive BMIs rely on the physiological property of individual cortical neurons to modulate their spiking activity in association with movements. These modulations are found to be highly variable from neuron to neuron and from trial to trial. Yet averaging across many trials reveals fairly consistent firing patterns. Based on the hypothesis that the function of neural circuits is an emergent property that arises from the coordinated activity of large numbers of neurons, this phenomenon can be explained. Individual neurons generally form synaptic connections with thousands of other neurons. In distributed circuits, the larger the connectivity matrix the greater the redundancy within the network. Given their distributed connections and their plasticity, neurons are likely to be subject to continuous dynamic rearrangement, participating at different times in different active ensembles. Accordingly both accuracy and reliability of predictions of motor activity improve considerably with increasing the number of simultaneously recorded neurons and decreasing the errors due to individual neuron firing variability. Pursuing this motivation, the number of simultaneously recorded neurons has been approximately doubling every 7 years since 1950’s. Standard recording techniques using 704 implantable micro wire arrays have been reported in literature. Recently Nicolelis Lab at Duke University announced their achievement to simultaneously record the electrical activity produced by a population of 1,874 interconnected single neurons at work in a primate.
Brain in a Dish:
At present, the prime methodology for studying neuronal extracellular activity under in vitro conditions is by using substrate-integrated microelectrode arrays (MEAs). This methodology permits simultaneous, long-term recordings (i.e. of up to several weeks) of extracellular field potentials. Correlating MEA recordings with microscopic imaging and stimulations is widely used to study the circuit-connectivity, dynamics and propagation effects in neuron assemblies. It is also used to investigate population coding, activity patterns, plasticity and pharmacological testing on either dissociated neuronal cultures or brain slices of embryonic rats, i.e. the young forests as Cajal described them. Commercially available MEA systems integrate typically 60–120 microelectrodes of 10–30 μm in diameter with pitches on the order of hundreds of micrometers. Typical neuron soma dimension in vertebrates is few micrometers long and the typical neuronal networks have 10000–50000 neurons, the limited number of electrodes and their rather large pitch results in a substantial spatial undersampling of the overall network activity as shown in Fig2.2.

Fig. 2.2: Substrate-integrated MEA dish. The microscopic image of the electrode (black) and neurons. Neural Instrumentation Lab, Temple University
The development of higher spatial and temporal resolution at low noise levels are prerequisites for opening the perspective to access the network electrical activity at the global and cell levels. Recently, CMOS-based high-density MEAs were developed featuring switching techniques to manage a large number of electrode channels interconnections, multiplexing, amplification, and filtering. Active Pixel Sensor based MEA platform providing 4096 microelectrodes at 21μm inter-electrode separation and 7.7KHz sampling rate has been documented. Considering the ultimate goal of Brain Activity Map, the current neuroscience in vivo and in vitro research states and the advancement of high density microelectrode arrays, the migration to monitoring thousands of recording channels at high temporal resolution is achievable.