Cancer Stem Cell Hypothesis
Stem cells are undifferentiated cells within a tissue that are functionally capable of indefinite division and self-renewal, thereby providing a regenerative pool for continuous tissue replenishment. Homeostatic regulations for cells within a tissue are maintained at the stem cell level. Following division, one of the two resulting cells will retain its undifferentiated, stem-like properties, while the other cell will have begun to differentiate into a progenitor cell. Unlike its predecessor, a progenitor cell loses the capacity to sustain indefinite division and will begin to express the properties and characteristics of the more mature, fully differentiated cells of the surrounding tissue type.
With regards to the multi-step model of carcinogenesis, wherein normal cells may become cancerous after sustaining several uncorrected mutations over multiple generations, it is unsurprising that loss of homeostatic regulation can cause normal adult stem cells to divide uncontrollably. These cancer stem cells, stem-like cells whose homeostatic mechanisms have been altered or subverted, can arise from either mutated adult stem cells or progenitor cells that have dedifferentiated back into stem cells. The CSC hypothesis maintains that these stem-like cancer cells are the basis of selfpropagating tumors: Small subpopulations of CSCs maintain growth of the larger tumor, with daughter cells differentiating into mature cells of the CSC tissue type with finite growth capacities. Experimental evidence strongly supports the existence of CSCs. Additional evidence suggests that hypoxic tissue conditions induce expression of certain transcription factors which contribute to tumor progression and may cause differentiated tumor cells to revert back into undifferentiated CSCs. The impact of tissue hypoxia on CSC populations makes its alleviation a high priority for the improvement of cancer therapy outcomes.
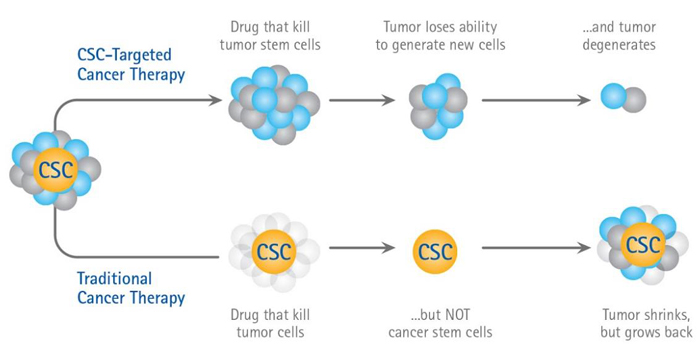
Cancer Stem Cell Hypothesis
CSC Biomarkers
Studies of tumor histology have provided a means of visualizing CSC populations. The effect of a drug, for example, on CSC populations can be monitored through quantifying the cells within a tumor volume that express specific CSC biomarkers. Multiple CSC biomarkers have been identified in previous experiments, including combinations of clusters of differentiation proteins, cell surface adhesion molecules and cytosolic enzymes.
One such biomarker is ALDH, a detoxifying enzyme that catalyzes the oxidation of aldehyde groups into carboxylic acids. Its ability to metabolize retinol into retinoic acid is under investigation for its role in stem cell differentiation. Found predominantly in liver cells due to its metabolic significance, overexpression has been associated with negative clinical prognosis for malignant human mammary stem cells.
ALDH expression on the exterior of the plasma membrane has been demonstrated in both CSCs and progenitor cells, while internal ALDH expression is strictly associated with the undifferentiated stem cells16. Antibody staining techniques may be utilized to identify ALDH expression on cell surfaces, while the ALDEFLUOR assay has been used to identify cells with cytosolic ALDH expression. EpCAM is a transmembrane glycoprotein found frequently on the basolateral surfaces of various epithelial cells, enabling cell-cell adhesion through calciumindependent pathways. Increased EpCAM activity correlates inversely with standard cadherin-mediated adhesion, which has the effect of deregulating epithelial cell growth and differentiation and increasing epithelial cell proliferation. EpCAM is being investigated as a potential target for cancer therapy due to its documented overexpression in a variety of carcinomas, including those originating in the pancreas, breast, prostate,
and colon1. DLL is a delta ligand homolog in humans that participates in multiple Notch signaling pathways: Ligands are passed from a signaling cell to the Notch cell surface domain, which initiates a series of cleavage events that in turn release transcription factors into the intracellular space. It is perhaps best known for its role in directing progenitor cell differentiation, promoting characteristics of T-cell precursors while blocking progression into B-cells19. Additionally, Notch signaling via DLL regulates stem cell renewal and differentiation in the lumen of normal breast tissue, a property that has been implicated in the initiation and progression of cancer cells.

