Antiangiogenic Therapy
Research into the treatment of cancers has yielded a multitude of different approaches and techniques. While a growing body of attention has focused on genomics for a more personalized experience, existing treatments most commonly include radiation exposure in conjunction with a carefully-planned regimen of cytotoxic chemical agents. Among those drugs considered for cancer treatment are a class referred to as angiogenic inhibitors, which attempt to stem tumor growth through the inhibition of blood vessel formation.
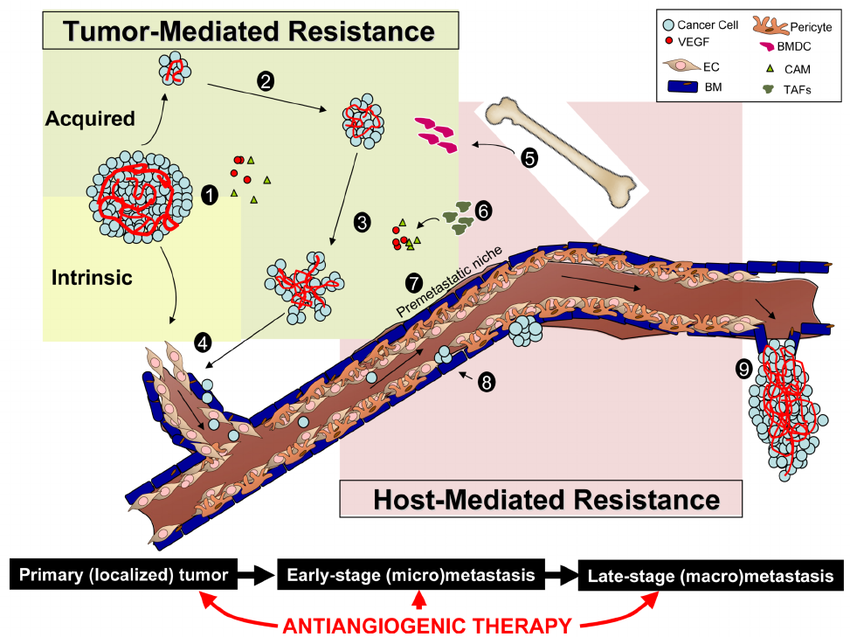
Angiogenesis is characterized in normal tissue by the formation of capillary micro vessels in response to migrating and proliferating endothelial cells. Regulated by a host of inhibitors and growth factors, angiogenesis is a critical component in wound repair, tissue development, and reproduction. While this process typically transpires over a selflimited period of weeks or months, pathology-linked angiogenesis can persist chronically for years. Such angiogenesis-dependent diseases include age-related macular degeneration, rheumatoid arthritis, atherosclerosis, and cancer; angiogenesis is critical to fueling the prolonged growth of neoplasms and their associated metastases.
Originally conceptualized in the late 1970s, antiangiogenic therapy was first theorized following the successful development of angiogenesis bioassays. In contrast to existing chemotherapy agents that target specific components of mitotic division, the theory behind AAT centers on the notion of cellular starvation: Tumors must sustain their constant expansion through the recruitment of new blood vessels, diverting oxygen and nutriment into their growing mass. By blocking blood vessel recruitment, it was theorized that sustained cell division would no longer be possible as supplies of glucose and oxygen were gradually exhausted. This alternative approach offered the potential for greater sparing of healthy tissue: While cell division-targeting drugs would exhibit cytotoxic effects on all dividing cells in the body, including normal cells, AAT drugs would only block the formation of new blood vessels, leaving existing vasculature intact.
Research into this technique has led to the development of a variety of functional AAT drugs, the earliest of which began clinical trials in the mid-1990s. Bevacizumab, a monoclonal antibody approved in 2004 for the treatment of colorectal cancer by the US Food and Drug Administration, was the first of these new drugs developed solely as an angiogenic inhibitor and is one of the better-studied of the emerging AAT agents. Marketed under the trade name Avastin, the drug disrupts the activity of vascular endothelial growth factor A (VEGF-A), one of the leading promoters of angiogenesis. Similar drugs such as the kinase inhibitor sunitinib and monoclonal antibody DC101 were developed to target VEGFR kinase activity and antigen binding respectively.
After years of studies accumulated, a common trend in AAT treatment of cancerous lesions manifested: While angiogenic inhibitors may provide positive clinical benefits against established tumor cell populations, they are not increasing overall patient survival. Bevacizumab was shown to confer no significant benefit to overall patient survival in cases of metastatic breast cancer, and both sunitinib and DC101 were shown to increase the rate of spontaneous metastasis in laboratory mice5-7 as well as increase long-term tumor invasiveness. These results suggest a relationship exists between AAT drugs and metastatic growth. One hypothesis purports that hypoxic conditions, which have already been implicated in decreased treatment effectiveness due to the decreased availability of reactive oxygen species8, are being worsened by exposure to angiogenic inhibitors. The tortuous and abnormal development of vasculature within cancerous lesions creates natural regions of chronic hypoxia, which are further intensified by rapid degeneration of tumor vasculature within the tumor mass. Such developments could inadvertently select for those cells which can survive under nutrient-starved conditions and therefore promote the growth of more aggressive cancer cells9,10. Additionally, extrapolating from gene regulation mechanisms identified in human embryogenesis, it is theorized that hypoxia may play a role in the activation of certain growth-specific gene expression pathways in cancer cells.
To address these circumstances, some researchers have pursued alternative AAT techniques: Instead of ablating blood vessel recruitment with high doses of angiogenic inhibitors, lower doses can be used to normalize tumor vasculature. Vasculature normalization can have the dual effect of increasing oxygen perfusion to the tumor mass,thereby alleviating hypoxic conditions and the various associated complications, and better allowing chemotherapy agents to access cancer cells within the tumor interior.
Antiangiogenic Therapy

